- Phone: 800-492-1252
- Fax: 440-368-3569
- E-mail: info@spartanwatertreatment.com
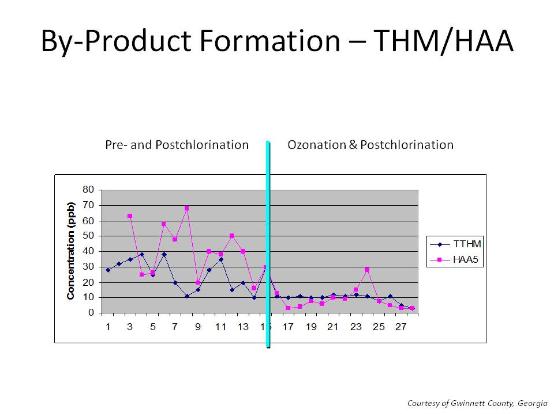
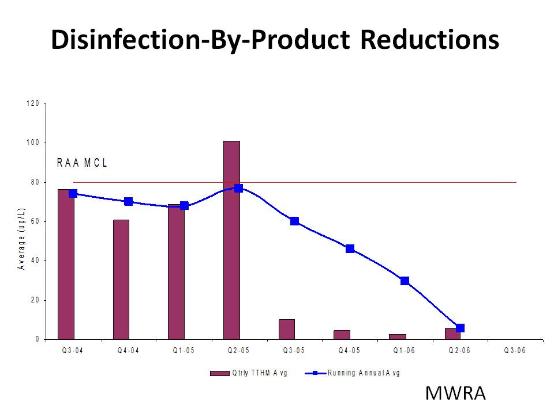
Chlorination of water with NOM and synthetic organic compounds can result in the formation of disinfection byproducts (DBP) such as trihalomethanes (THM) and haloacetic acids (HAA). This occurs due the chlorination of the organic material in the water to THM and HAA.
These compounds are regulated in drinking water treatment to levels below 80 ppb. The Stage 2 DBP rules recently promulgated by the EPA will result in more stringent enforcement by changing the how these levels are measured and calculated. At the same time, the rules for treating drinking water for micro organisms such as Giardia and Cryptosporidium (LT2ESWTR) are becoming stricter requiring greater disinfection capabilities. This means that drinking water plants will need to use alternative disinfectants or barriers to meet both objectives.
Ozone has been shown to significantly reduce DBP formation if properly applied. Ozone can reduce DBP in several ways:
First, it can reduce the amount of chlorine required by providing some of the disinfection credits needed to treat the water. In fact, ozone is a better disinfectant than chlorine against many micro organisms. For example, ozone is 100 times more effective against Giardia than chlorine. Using ozone as preoxidant or as the primary disinfectant can significantly lower the amount of chlorine required as a secondary disinfectant for the distribution system.
Second, in conjunction with biologically active filters , ozone can significantly reduce the amount of organic material available to be chlorinated and thus form THM or HAA. Biological filters can also help meet TOC targets in the finished water. Biologically active filters are normal filters where biology is allowed to develop. The organisms in the filter than degrade and remove organic material from the water. Ozone aids this process. The charts below show the impact of ozone/biofiltration on THM levels at two large drinking water authorities before and after the introduction of ozone:
Ozone can be effective in partially oxidizing organics in the water to biodegradable compounds that can be removed by biological filtration. This partial oxidation gives rise to lower molecular weight organics that are more easily biodegradable. This increase in the biodegradable fraction of organic carbon occurs as a result of moderate to high levels of ozonation. These ozone levels are typical of the doses commonly applied for disinfection.
Ozonation typically increases the biodegradability of NOM in water because many large organic molecules are converted into smaller organic molecules that are readily biodegradable. This increase in biodegradable dissolved organic carbon (BDOC) can lead to accelerated bacterial growth and regrowth in the distribution system if not removed in the treatment plant. It has been found that AOC levels less than 100 ppb may be necessary to control excessive bacterial regrowth in the distribution system if not removed in the treatment plant. When ozonation is placed upstream of filtration, and environmental conditions such as dissolved oxygen, pH, and temperature are favorable, microbiological activity is increased in the filter and BDOC/AOC removal is enhanced. Ozone addition not only increases the biodegradability of the dissolved organics, but also introduces large amounts of oxygen to the water, thus, creating an excellent environment for biological growth on the filter media. The advantages of biologically active filtration include the following which are all being met in most US plants using ozone:
Biological activity can be supported on slow sand, rapid rate, and GAC media because these media provide a surface for bacteria to attach. Factors such as available surface area, hydraulic loading rate, contact time, availability of nutrients, temperature, and others will determine the performance and BDOC removal efficiency. Biomass develops to higher levels on GAC because of the rougher surface characteristics than on anthracite and sand.
Ozone addition prior to slow sand filtration can increase the efficiency of TOC removal by about 35 percent. Ozone addition can also increase the efficiency of BDOC removal with slow sand filters.
Research in the area of biologically active rapid rate filters has focused on the reduction of assimilable organic carbon (AOC) instead of BDOC. While studies have shown rapid rate filtration, employing either sand or dual media lowers AOC levels following ozonation, AOC does not measure all the BDOC. AOC measures only that portion of the BDOC that is more easily assimilable or more easily biodegradable under specific laboratory conditions by two specific microorganisms. Research data shows that biodegradation of AOC can occur in rapid rate filters. The data should be viewed with caution, since the more slowly biodegradable DOC, not measured by AOC, may be passing through rapid rate filters.
Activated carbon is made biologically active by the deliberate introduction of sufficient dissolved oxygen to water just before passing through GAC columns. The high surface area and long retention time in GAC provide an ideal environment to enhance biological growth. Although ozone actually increases the amount of BDOC, the efficiency of subsequent biodegradation on GAC can be such that the BDOC in GAC effluent is lower than the BDOC in the ozone influent. The degree to which biodegradable DOC is removed by ozone/GAC depends upon the process conditions of temperature, amount of BDOC, and the GAC column loading rate, measured by empty bed contact time (EBCT). For example, with an influent BDOC of 0.65 mg C/L and a 10 minute EBCT, one would expect an effluent BDOC of 0.25 mg C/L. The effluent BDOC then could be lowered by either:
Without biologically active filters or operating under the wrong condition, the benefits of ozone may not be obtained. The key variables that seem to determine ozone’s effect are dose, pH, alkalinity, and, above all, the nature of the organic material. At low pH levels, precursor destruction by ozone is quite effective; however, above some critical pH, ozone actually is less effective and in fact sometimes increases the amount of chlorination byproduct precursors.
For most humic substances this critical pH is 7.5, which is the approximate level at which decomposition of ozone to hydroxyl free radicals increases rapidly, thus increasing organic oxidation rates. Therefore, the implications that at lower pH approximately 6-7), at which molecular ozone predominates over the hydroxyl free radical, the initial THM precursor byproducts are different in nature than those formed by the hydroxyl free radicals oxidized at higher pH levels. This is logical in light of the greater oxidation potential of the hydroxyl free radical over that of ozone. As alkalinity increases, it has a beneficial effect on THM formation potential (THMFP).
This is because alkalinity scavenges any hydroxyl free radicals formed during ozonation, leaving molecular ozone as the sole oxidant, which is only capable of oxidizing organic precursors to a lower oxidation sequence than does the hydroxyl free radical. Given neutral pH and moderate levels of bicarbonate alkalinity, THMFP level reductions of 3 to 20 percent have been shown at ozone doses ranging from 0.2 to 1.6 mg ozone per mg carbon.
Ozone water treatment can lead to the bromate formation which is regualted by the EPA to less than 10 ppb. If properly applied, bromate levels can be kept below this level except for the most difficult disinfection and water quality situations.
Huck, P.M., P.M. Fedorak, and W.B. Anderson. 1991. “Formation and Removal of Assimilable Organic Carbon During Biological Treatment.” J. AWWA. 83(12):69- 80.